The clinical Pharmacology and Pharmacometrics office (CPPO) was established in January, 2019, and mainly includes the following five types of research: modeling and simulation, data management and statistics, GLP bioanalysis and translational research laboratory (including Shimadzu Drug Metabolism Innovation Center), intelligent system development laboratory, and Peking University Third Hospital (PUTH)-Leadingpharm Medical Technology Joint Research and Development Center for Modified New Drugs Development (hereinafter referred to as the Joint R&D Center). At present, CPPO has 20 full-time researchers, including 1 professor and 2 associate professors, 6 teaching and research staffs, 55% of whom have doctoral degrees and 95% have graduate degrees; At the same time, there are 14 postdoctoral and postgraduate students.
The purpose of CPPO is to comprehensively utilize the technologies of clinical pharmacology, pharmacometrics, statistical analysis, bioanalysis, multi-omics analysis to integrate pharmacokinetic/pharmacodynamic/disease information. CPPO together with departments of PUTH and domestic and foreign colleagues, and carry out collaborative innovation to support a new drug development pattern establishment, regulatory science innovation and clinical efficacy improvement informed by a “All Life Cycle” model from pre-clinical, clinical trials, to post-marketing, ultimately improving drug development efficiency, improving therapeutic effect, developing good drugs with good quality and low price for Chinese patients, and exploring the optimal therapeutic schedule. The research of CPPO focuses on the new drug development for endocrine and metabolic diseases, cardiovascular diseases, kidney and tumor targeted therapies, and the intelligent development of improved new drugs.
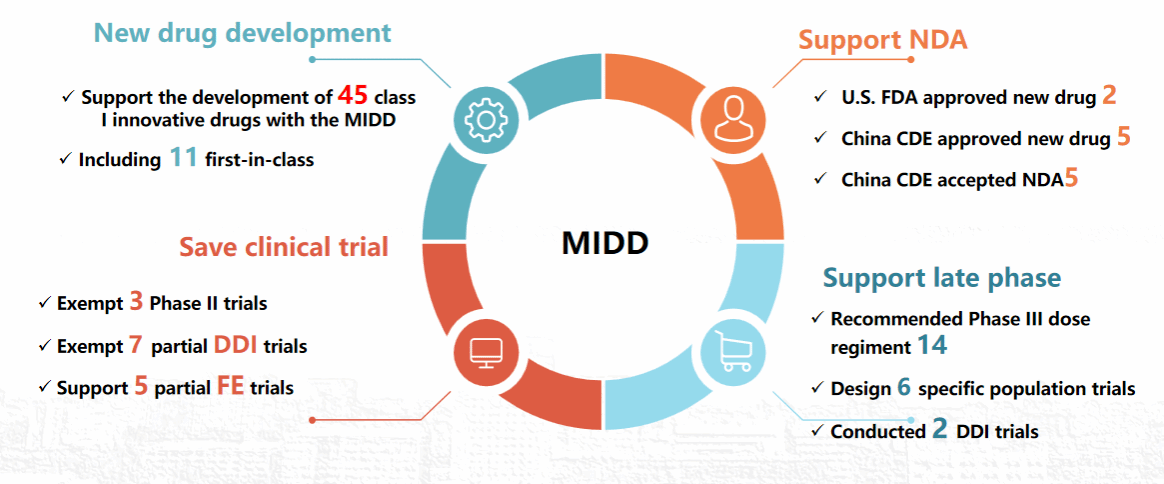
Within the above research orientation, CPPO has received 7 national fundings in the past four years, including 5 National Natural Science Foundation of China and 2 sub-projects of Major Special Projects of the Ministry of Science and Technology; CPPO has received 2 provincial fundings and 2 fundings from the Bill and Melinda Gates Foundation. With the above-mentioned financial support, CPPO recommended new precision medicine strategies for six clinical first-line drugs, recommended optimization strategy of drug administration in emergency, and accelerated the clinical development of 45 new moiety entities with the model informed drug development (MIDD) strategy. Two drugs were approved by the United States Food and Drug Administration, another 5 drugs were approved by China National Medical Products Administration, and 5 drugs were supported to complete phase III clinical trials and are ready to submit or have submitted New Drug Application. And a total of 3 new drugs were exempted from phase II clinical trials, 7 new drugs were exempted drug-drug interaction clinical trials and 5 new drugs were exempted food effect clinical trials, which had direct social benefits of hundreds of millions (see the figure below for details).
CPPO have published more than 50 SCI articles as the first author or corresponding author (including the co-corresponding author) in the past four years, including 7 papers ranked in the Medicine zone 1, Chinese Academy of Sciences (Acta Pharmaceutica Sinica B, LANCET: EclinicalMedicine, Clinical Infectious Diseases, Sci China Life Sci), 2 papers ranked in the Pharmacy zone 1 (Clinical Pharmacokinetics). The total citation is over 3000. CPPO won one software monograph patent and won the medal of the second National Innovation Award (the sixth) and the award of "Advanced Collective and Advanced Individual of the National Science and Technology System in Fighting Against the Epidemic of COVID-19".
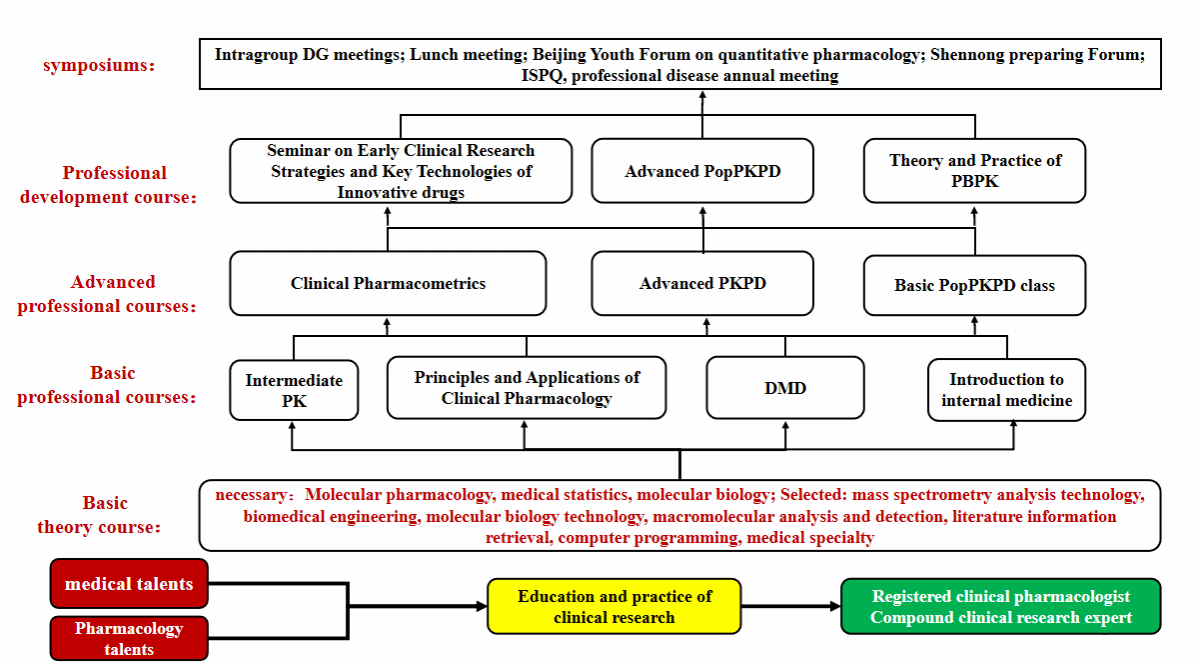
CPPO also paid special attention to pharmacometrics education. CPPO established a pharmacometrics training system including 10 courses (see the figure below for details), providing high-quality learning opportunities for domestic researchers and students. These courses contain basic content and advanced application, and more than 1000 trainees have been trained. Five courses in which more than 70% teaching tasks are undertook by CPPO staffs, include 2 postgraduate courses (Principles and Applications of Clinical Pharmacology,Clinical Pharmacometrics) in the Department of Medicine of Peking University, one summer camp course for undergraduate (Intermediate Pharmacokinetics) and 2 national continuing education courses (Basic Seminar on PopPKPD model Analysis, Theory and Practice of PBPK). Experts at home and abroad are invited to teach three continuing education courses (Advanced PKPD, Advanced PopPK and Seminar on Early Clinical Research Strategies and Key Technologies of Innovative drugs). And Beijing Youth Forum on Pharmacometrics (ByFP)and ShenNong Preaching Forum (问道神农论坛)are regularly hosted. At present, CPPO preparations a textbook 《Clinical Pharmacokinetics and Pharmacodynamics Research: Basic and Frontier》, and has participated in editing chapters related to pharmacometrics and clinical development of new drugs in four textbooks《Pharmacokinetic Theory and Practice》, 《Biopharmaceutics and Pharmacokinetics》, 《Clinical Pharmacokinetics》 and 《Clinical Pharmacokinetic》.
1、Research capabilities
1) Modeling and simulation research for the whole life cycle of new drugs
CPPO equips with various modeling and simulation software (NONMEM/R/PIRANA, SimCYP (Authorized to provide registration services for Chinese enterprises), Phoenix NLME, MATLAB, Berkeley Madonna, ADAPT5, ADMET predictor), clinical pharmacology analysis software (certified Phoenix WNL), drug discovery and docking software (AutoDock, VINA, DOCK6), and multiple high-end servers. CPPO has the software and hardware conditions to carry out the whole life cycle modeling and simulation for new drugs and can support the clinical pharmacology and pharmacometrics research of new drug registration. CPPO can provide first-in-human dose prediction (FIH), population pharmacokinetics pharmacodynamics (PPKPD) analysis, physiologically-based pharmacokinetics (PBPK) analysis, exposure-response analysis (ER), model-based meta-analysis (MBMA), meta-analysis (MA), quantitative system pharmacology analysis (QSP), real-time data analysis (RTDA) in drug development, clinical trial scheme design, clinical study report writing, clinical pharmacology report writing and other technical services.
2) Data management and statistics of clinical pharmacology studies
CPPO equips with statistical software SAS, R and clinical pharmacology software Phoenix WinNonlin, which can support the statistical analysis of pharmacokinetic, pharmacodynamic, safety inspection data and ER analysis of phase I and phase II clinical trials. At present, CPPO has completed several clinical statistical analysis for single ascending dose, multiple ascending dose, food effects, and drug-drug interaction clinical trials of first-in-class new drugs. CPPO also provides clinical data management based on the Peking University Health Care Big Data Platform and several computing servers.
3) GLP bioanalysis and multi-omics research for new drugs development
CPPO has Meso Scale Discovery (MSD) electrochemiluminescence detection system, Molecular Devices SpectraMax iD3, multifunctional enzyme labelling instrument (equipped with SoftMax Pro GxP compliance software), liquid chromatography-mass spectrometer (Shimadzu UFLCMS-8060NX), ultra performance liquid chromatography coupled to quadrupole time of flight spectrometer (ExPEC 5600), Watson LIMS laboratory information management system, and labwatch temperature control system. Under the cooperation with Shimadzu Drug Metabolism Innovation Center, CPPO obtained the license for the comprehensive cooperation of series of mass spectrometry innovative instruments of Shimadzu China Innovation Center. With the full support of the Basic Medical Center of PUTH and the State Key Laboratory of School of Pharmacy, Peking University, CPPO fully meets the hardware and software requirements for small molecule and macromolecular new drug biological detection, metabolites in safety testing (MIST), biomarker development, and pharmacological mechanism exploration. At present, CPPO has completed the biological sample analysis for 3 new drugs, including drug concentration, biomarkers, and anti-drug antibodies methods development and detection. CPPO has conducted multiple MIST, new drugs metabolomics studies, enzyme and transporters targeted proteomics and single-cell sequencing and analysis studies related to new drug mechanisms.
4) Pharmacometrics MIDD and MIPD research software development
With the support of external experts, CPPO has a software development team composed of algorithm engineers, product managers, software development engineers, and patent experts, as well as multiple high-performance servers, which can support the entire process of pharmacometrics MIDD and model informed precision medicine (MIPD) research from demand to product translational services. Software development engineers have the ability and experience to develop desktop software based on C# language and WPF framework, as well as mobile software based on JAVA language and MVP, MVVM front-end and back-end frameworks in multiple languages and platforms.
5) Intelligent development of modified new drugs
In 2022, with the support of hospital leaders, PUTH-Leadingpharm Medical Technology Joint Research and Development Center for Modified New Drugs Development was established in cooperation with Beijing Leadingpharm Technology Co., Ltd.. Focusing on the "new mode for modified new drugs intelligent development ", the Joint R&D Center conducts in-depth research on cutting-edge drug delivery technology, AI simulation data modeling, and the application of in vivo and in vitro PK/PD bridging and other technologies in new drug research and development. The Joint R&D Center will be able to achieve efficient transformation and innovation of research mode, and take children drugs, cancer and other improved new drugs as experimental fields, guided by unmet clinical needs, integrate new technologies such as modeling and simulation, domestic and international cutting-edge formulation development, and gather the forces of clinical doctors, academic committees, Leadingpharm and Drug Clinical Trial Center to explore and incubate new products, technologies, and strategies, enhance development and transformation capabilities, and clinical discipline innovation capabilities, and jointly empower and accelerate the innovative development of improved new drugs in China, propose efficient and effective comprehensive solutions for the mission of addressing the global patient's "people-oriented, unmet clinical needs of precision medicine".
6) Non-registrational exploratory research
In order to better support the clinical development of new drugs, CPPO fully cooperates with the sponsor and basic research partners, and carry out non registered exploratory research, including investigator initiate trials (such as the research on pharmacokinetics and pharmacodynamics of specific populations and the research on the regulation mechanism of critical metabolic enzymes and transporters) , efficacy or toxicity mechanisms research of new drugs (such as the study of glucokinase agonists in improving impaired glucose tolerance, the study of the renal toxicity mechanism of URAT1 inhibitors, the clinical pharmacology study of Yunnan Baiyao in improving fracture pain and treating fracture healing, etc.), in order to efficiently solve the problems encountered in the development of clinical drugs and provide clues for the potential clinical value of new drugs.
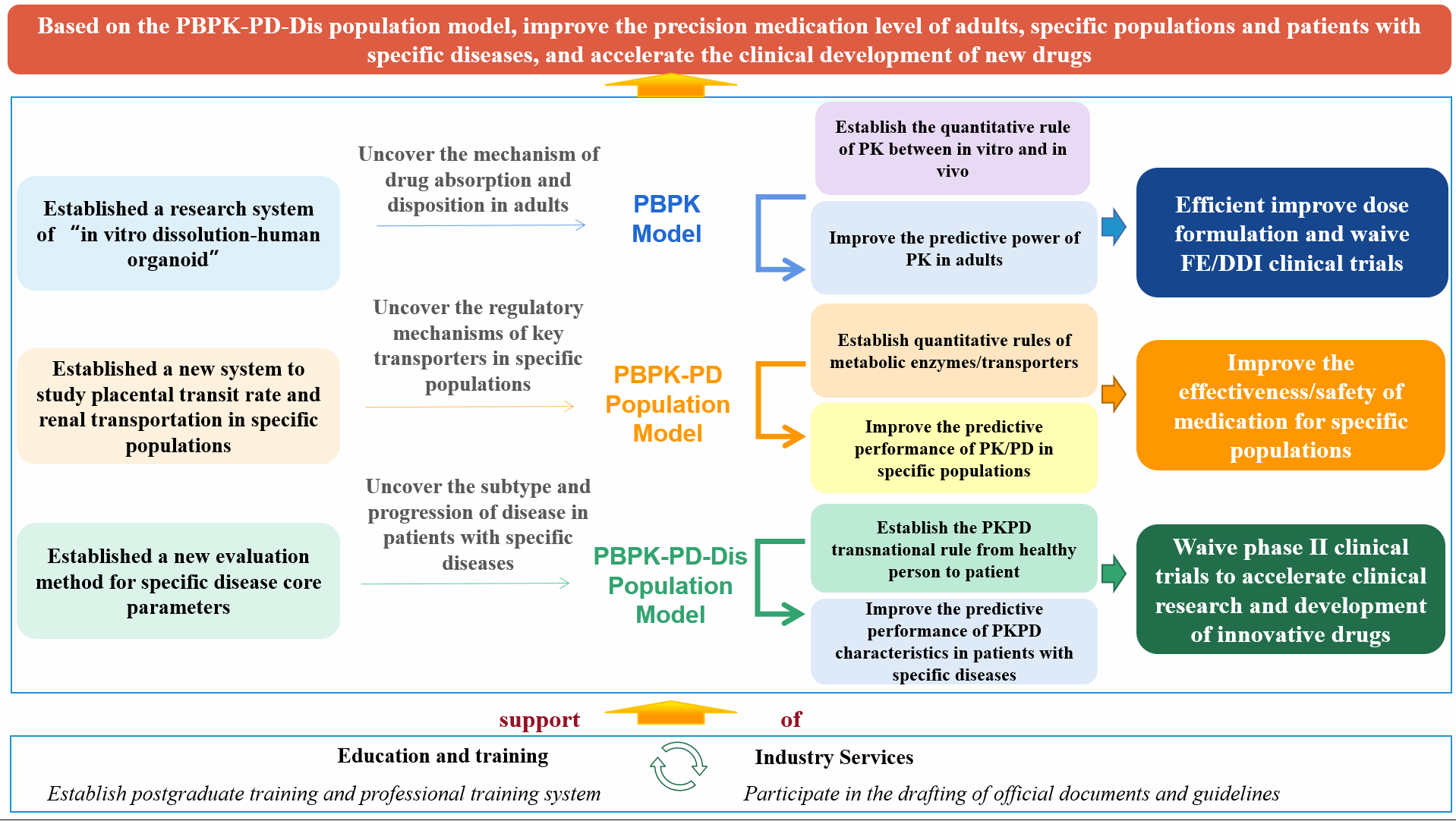
2. Research characteristics(in vitro and in vivo experiments - PBPK/PD/Dis model "integrated prediction system")
1) Established a research system of "in vitro dissolution-human intestinal organoid" to reveal new regulatory mechanisms of oral drugs dissolution in the intestine, establish quantitative correlation between in vitro and in vivo, and a PBPK model new predicting strategy, significantly improving prediction accuracy. Taking the lead in proposing a new strategy of “exploring food effect and exempting clinical trials of new drugs ", leading implementation of the drug-drug interactions clinical trials exemption based on PBPK model, guided 28 new drugs development (over 10 disease fields).
2) Established the in vitro and in vivo research system of maternal placental trophoblast cells - human placenta perfusion, human renal tubular epithelial cells / HK-2 cells - human kidney sections and juvenile rat - premature / newborn and analysis system of key metabolic enzymes and transporters in human tissues (based on the endogenous markers, exosome analysis and multi-omics technology) to reveal the functional levels, regulatory mechanisms and significant influencing factors of metabolic enzymes and transporters in tissues of pregnant women, children, the elderly and people with renal injury, establish dose-concentration quantitative relationship. A total of six Chinese specific population PBPK models were established, four of which could cover more than 50% drug disposal pathways, significantly improving the predictive performance of pharmacokinetic characteristics in specific populations, reducing the risk of drug safety or effectively expanding new indications.
3) Established a new calculation method of core indicators of disease (insulin secretion ability and sensitivity of diabetes), established a diabetic patients PBPK model to reveal the changes of the core indicators of endocrine and metabolic diseases and key influencing factors, and thus established a dose-exposure-response quantitative relationship. Based on the PBPK-PD-disease progression model, CPPO established a new strategy of accelerated clinical development of new drugs for metabolic, cardiovascular disease and cancer, and guided 16 first-in-class new drugs.
 ENGLISH
ENGLISH